mhysterie.ouahpiti.info
PRATIQUE Les Infections Sexuellement Transmises Patrick Olombel Professeur associé de médecine générale : UFR Rouen Résumé : La recrudescence des Infections Sexuellement Transmises (IST) comme la gonococ- cie et la syphilis, en France et dans la plupart des pays occidentaux, témoigne d'une augmenta- tion des rapports non protégés. Une IST diagnostiquée précocement et donc t

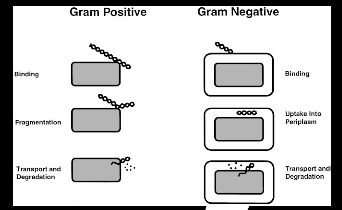 Figure 4.1: Transformation pathways in gram-positive and gram-negative
Figure 4.1: Transformation pathways in gram-positive and gram-negative 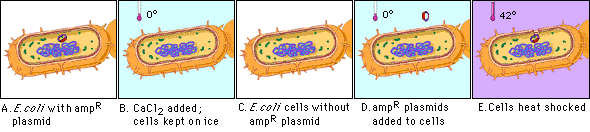 This method works well for circular plasmid DNAs but not for linear molecules such as fragments of chromosomal DNA. Figure 4.2: Overview of competence and heat shock
From: http://www.phschool.com/science/biology_place/labbench/lab6/test1.html
This method works well for circular plasmid DNAs but not for linear molecules such as fragments of chromosomal DNA. Figure 4.2: Overview of competence and heat shock
From: http://www.phschool.com/science/biology_place/labbench/lab6/test1.html